[Publication] Methodological Considerations for Setting Up Human-Relevant In Vitro Nanotoxicology Experiments—A Practical Guide
Abstract
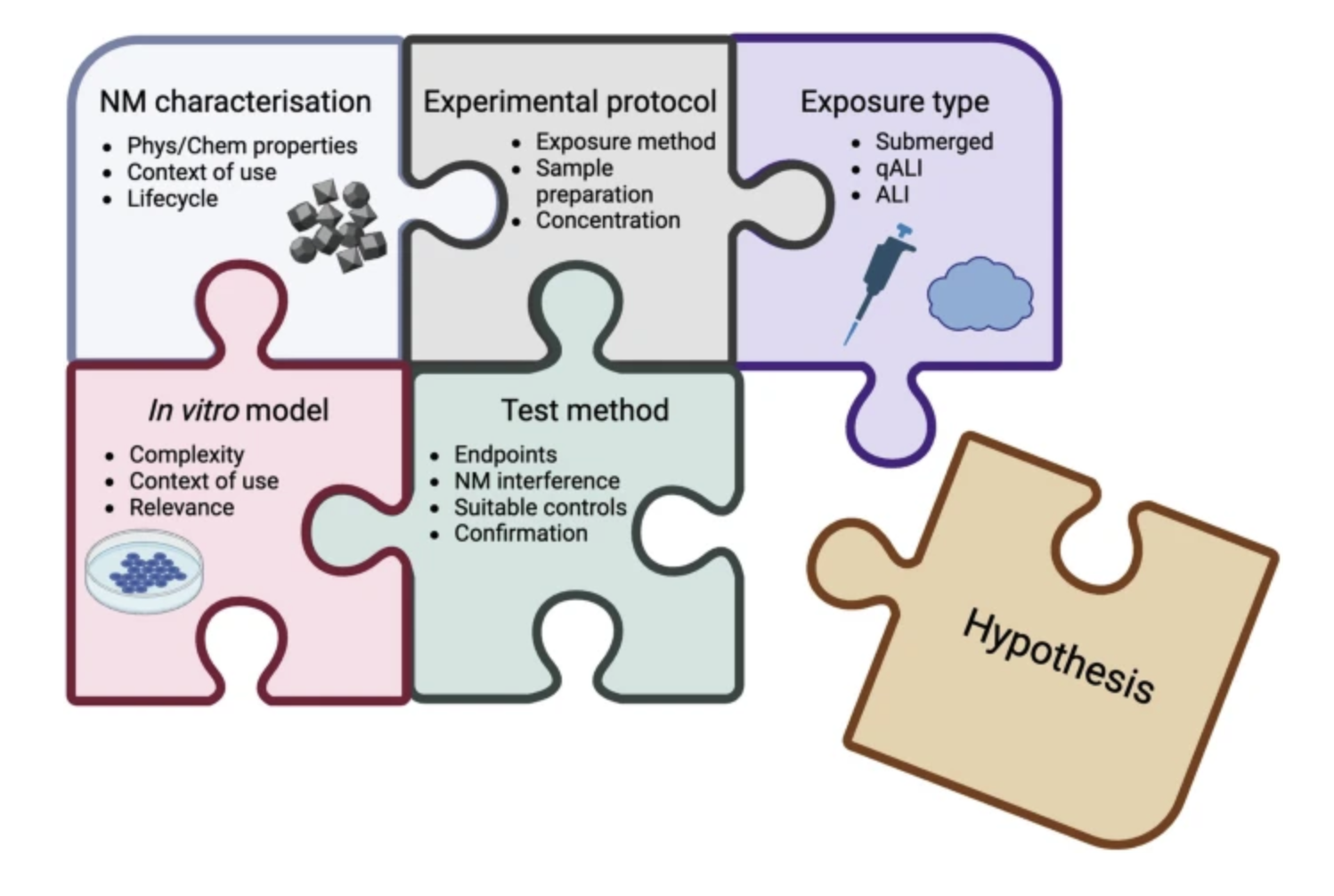
Nanotoxicology is a rapidly evolving field dedicated to assessing the safety and potential hazards of nanomaterials on human health. This practical guide outlines essential methodological considerations for designing human-relevant in vitro nanotoxicology experiments. A primary focus is placed on the comprehensive characterization of the nanomaterial in question, as properties such as size, shape, surface charge, and solubility significantly influence biological activity. The guide discusses the selection of appropriate in vitro models, including various cell sources, to ensure relevance to human exposure scenarios.
It is crucial to exercise caution when choosing test methods to account for potential nanoparticle interference with the selected assays; however, the use of suitable controls can help mitigate the impact of these interactions. The guide also emphasizes accurate practices for nanomaterial sample preparation and the importance of dosimetry, facilitating the translation of in vitro findings to realistic human exposure conditions. Guidance on exposure concentrations is provided to ensure that testing remains biologically and environmentally relevant. Furthermore, the guide includes reflections and perspectives on addressing common challenges and enhancing reproducibility in nanotoxicology studies. By adhering to these guidelines, researchers can generate more reliable and human-relevant in vitro nanotoxicology data, thereby supporting the risk assessment of nanomaterials.
Reference: Vilas-Boas, V., Arnesdotter, E., Carvalho, F., Alfaro-Moreno, E. (2025). Methodological Considerations for Setting Up Human-Relevant In Vitro Nanotoxicology Experiments—A Practical Guide. In: Alfaro-Moreno, E., Murphy, F. (eds) Nanosafety. Springer, Cham.